
[ad_1]
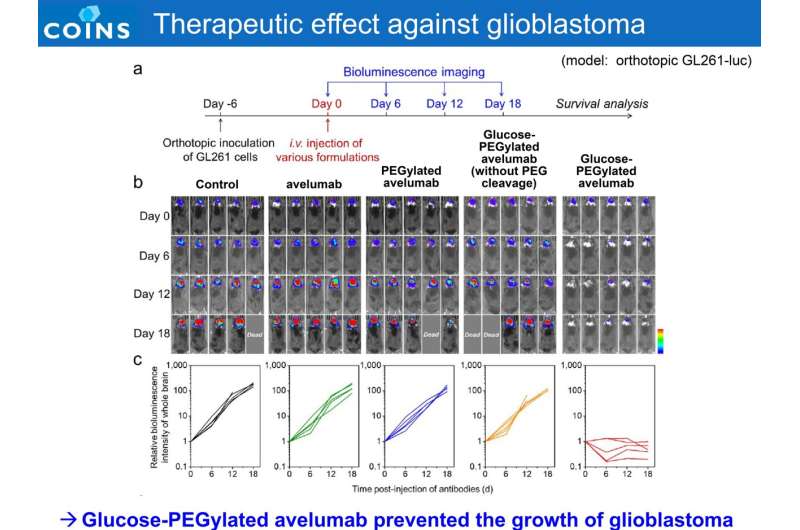
Multi-institutional researchers have succeeded in efficiently delivering an immune checkpoint inhibitor (ICI) into the mouse brain, confirming its high efficacy and specificity in treating orthotopically transplanted mice with glioblastoma (GBM). The research was published in Nature Biomedical Engineering.
Immune checkpoints are a normal part of the immune system, preventing an immune response from being so strong that it destroys healthy cells in the body. Immune checkpoints engage when proteins on the surface of immune cells called T cells recognize and bind to partner proteins on other cells, such as some tumor cells. For this discovery, Professor Tasuku Honjo of Kyoto University was awarded the Nobel Prize in Physiology or Medicine in 2018; six types of ICIs are now used in clinical practice for cancer treatment. However, while ICIs have shown excellent efficacy against a variety of cancers, they have not shown satisfactory efficacy in clinical trials against malignant brain tumors (including brain metastases). One of the reasons for this is that the blood-tumor barrier formed by the blood vessel walls of brain tumors suppresses the accumulation of immune checkpoint inhibitors in brain tumors. On the other hand, ICI formulations possess dose-limiting toxicity, eventually leading to life-threatening immune-related adverse events.
Hence, the goal of this study was to develop a potent technology to increase the accumulation of ICIs in brain tumors, and to achieve both therapeutic efficacy and safety. Avelumab, which has been shown to be immunologically active in both humans and animals, was used as an ICI. As glucose transporter 1 (GLUT1) are over-expressed on brain capillary and GBM vasculature to support the sufficient energy uptake, the ICIs with properly configured glucose molecules recognized the GLUT1 in GBM vasculature to promote access into GBM with ~20-fold accumulation, as compared to native antibodies.
Furthermore, the amount of ICI accumulated in the brain tumor site was 33 times higher than that in normal brain tissue, indicating high brain tumor selectivity. Taking advantage of the reductive environment of GBM with elevated extracellular GSH or ascorbate levels that are up-regulated by GSH-dependent enzyme (e.g., glutathione-S-transferases) and hypoxic microenvironment, the specificity of Glc-ICIs was improved via introduction of disulfide bond as the linker.
The ICIs formulation retrieved their PD-L1 blocking ability in tumor tissues via PEG chain detachment, while their activity remained muted in healthy tissues. Through efficient delivery and specific immune response, the modified antibodies achieved potent anti-tumor efficacy against a preclinical GBM model, which is a challenge for ICIs in a clinical setting (60% complete response rate in a mouse orthotopic GBM model). Furthermore, the drug was administered only once, with a low dose (1.5 mg/kg administered once in this study, compared to the standard drug dose of 10 mg/kg administered multiple times) and was found to be sufficient to achieve the desired effect.
-
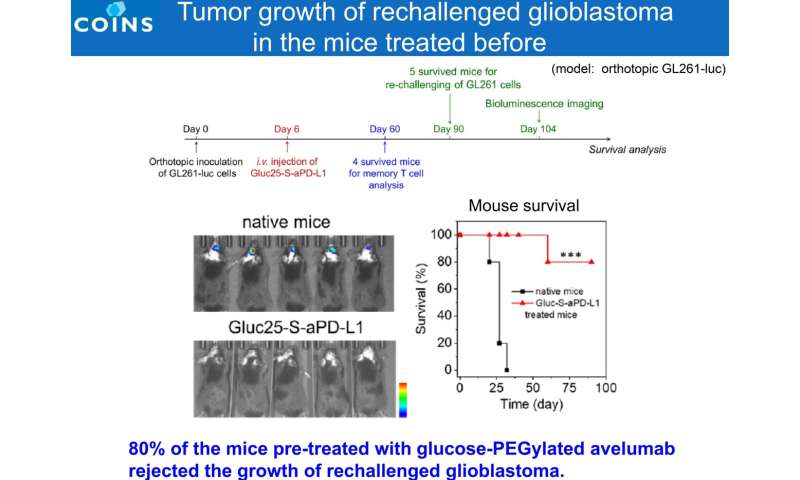
80% of the mice pre-treated with glucose-PEGylated avelumab rejected the growth of rechallenged glioblastoma. Credit: 2021 Innovation Center of NanoMedicine
-
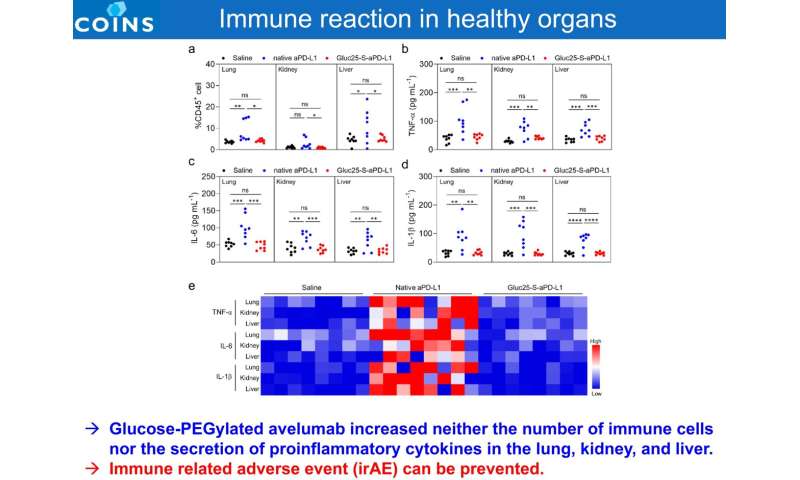
Glucose-PEGylated avelumab increased neither the number of immune cells nor the secretion of proinflammatory cytokines in the lung, kidney, and liver. Immune related adverse event (irAE) can be prevented. Credit: 2021 Innovation Center of NanoMedicine
Upon examination of antitumor immune cells in brain tumors of mice treated with Glc-ICI, it was shown that the number of tumor-attacking natural killer (NK) cells and CD8+ T cells increased, along with the effective re-polarization of M2-like macrophages to M1-like macrophages (an antitumor state). Meanwhile, the immunosuppressive microenvironment was also reshaped with declined population of regulatory T cells (Treg) and bone marrow-derived immunosuppressive cells (MDSCs). In addition, effector memory T cells were found in the spleen of mice that responded to Glc-ICI and whose tumors disappeared within 60 days (complete response), leading to a total rejection of tumor relapse. Although GBM is a malignant tumor that recurs frequently, these results are expected to be applicable to the prevention of GBM recurrence.
Horacio Cabral, Conjugation of glucosylated polymer chains to checkpoint blockade antibodies augments their efficacy and specificity for glioblastoma, Nature Biomedical Engineering (2021). DOI: 10.1038/s41551-021-00803-z. www.nature.com/articles/s41551-021-00803-z
Provided by
Innovation Center of NanoMedicine
Citation:
Antibody delivery technology empowers immunotherapy against glioblastoma and suppresses side effects (2021, October 11)
retrieved 11 October 2021
from https://medicalxpress.com/news/2021-10-antibody-delivery-technology-empowers-immunotherapy.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
[ad_2]
Source link