
[ad_1]
By Intratec Solutions |
This column is based on “Cumene from Benzene and RG Propylene (Zeolite Catalyst) – Cost Analysis,” a report published by Intratec. It can be found at: www.intratec.us/analysis/cumene-production-cost.
Cumene (also known as isopropylbenzene) is an organic compound of the aromatic hydrocarbon class. It is a colorless liquid, insoluble in water, but soluble in most organic solvents. The production of cumene grew rapidly during World War II, when this aromatic compound was used as a component of aviation fuel.
Today, cumene is mainly employed as an organic solvent in pharmaceutical manufacturing and as a flow-control agent for thermoplastic elastomer processing. Cumene derivatives (mainly phenol, acetone, and methyl styrene) are compounds with many chemical and industrial uses. Currently, the production of cumene is largely associated with the demand for phenol and phenol derivatives.
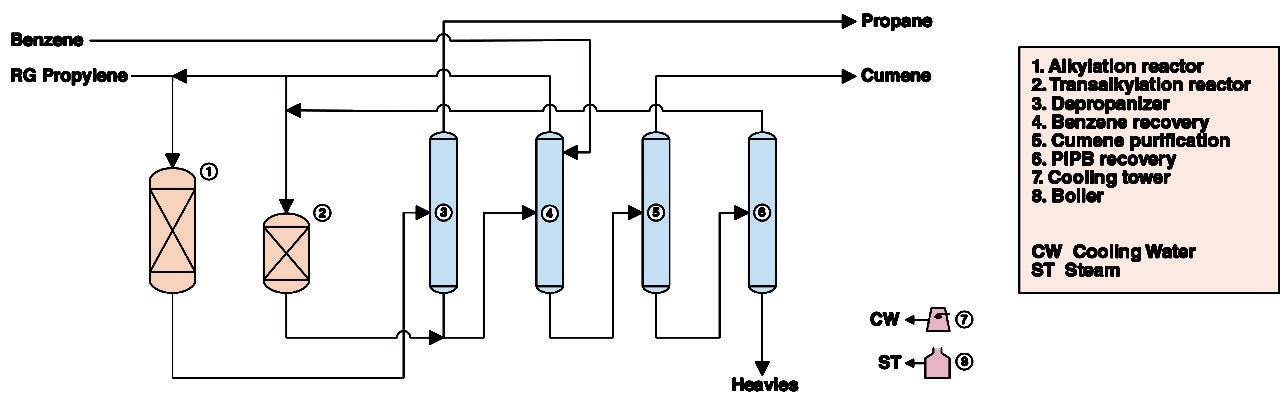
Figure 1. The diagram show cumene production from benzene and propylene
The process
The present analysis discusses an industrial process for cumene production. The process has two main sections: (1) reaction; and (2) distillation.
Reaction. In the reaction section, there are two reactors: the alkylation reactor and the transalkylation reactor. Fresh refinery-grade (RG) propylene and recycled benzene are mixed and fed to the alkylation reactor. In addition to the cumene product, the alkylation reactor effluent comprises unreacted benzene, non-reactive propane (present in the propylene feed), side products polyisopropylebenzenes (PIPB) and heavy ends. The effluent is routed to the depropanizer column in the distillation section. The transalkylation reactor, in turn, is fed with recycled benzene and PIPB recovered in the distillation section. In the transalkylation reaction, PIPBs are reacted with benzene to form cumene. The transalkylation reactor effluent is routed to the benzene recovery column in the distillation section.
Distillation. In the distillation section, a depropanizer column removes non-reactive propane from the alkylation reactor effluent. The propane-free reaction effluent is routed to the benzene-recovery column, while propane is purged from the process. The benzene-recovery column is also fed with fresh benzene and the product of the transalkylation reactor. Benzene is separated and recycled to the reaction section of the process, while a crude cumene stream, obtained from the bottom of the column, is routed to the cumene-purification column, from which high-purity product is obtained. The impurities rejected in the cumene column are treated in a further column, which recovers PIPB for the transalkylation reaction.
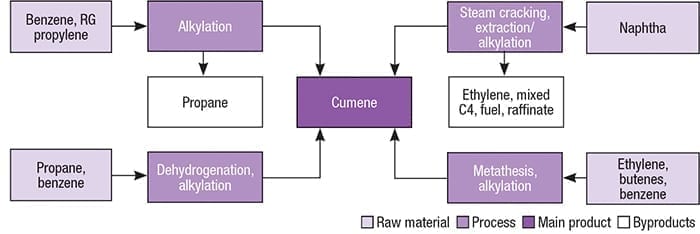
Figure 2. Multiple pathways exist for cumene production
Production pathways
The two main starting materials for cumene manufacture are benzene and propylene. Benzene and propylene are reacted via alkylation to produce cumene. Polyisopropylbenzene byproducts can be reacted with additional benzene via transalkylation to enhance cumene yield. Figure 2 presents different pathways for the production of cumene.
Economic performance
The total operating cost (raw materials, utilities, fixed costs and depreciation costs) estimated to produce cumene was about $750 per ton of cumene in the third quarter of 2016. The analysis was based on a plant constructed in the U.S. with the capacity to produce 300,000 metric tons per year of cumene.
Edited by Scott Jenkins
Editor’s note: The content for this column is supplied by Intratec Solutions LLC (Houston; www.intratec.us) and edited by Chemical Engineering. The analyses and models presented are prepared on the basis of publicly available and non-confidential information. The content represents the opinions of Intratec only. More information about the methodology for preparing analysis can be found, along with terms of use, at www.intratec.us/che.
[ad_2]
Source link