
[ad_1]
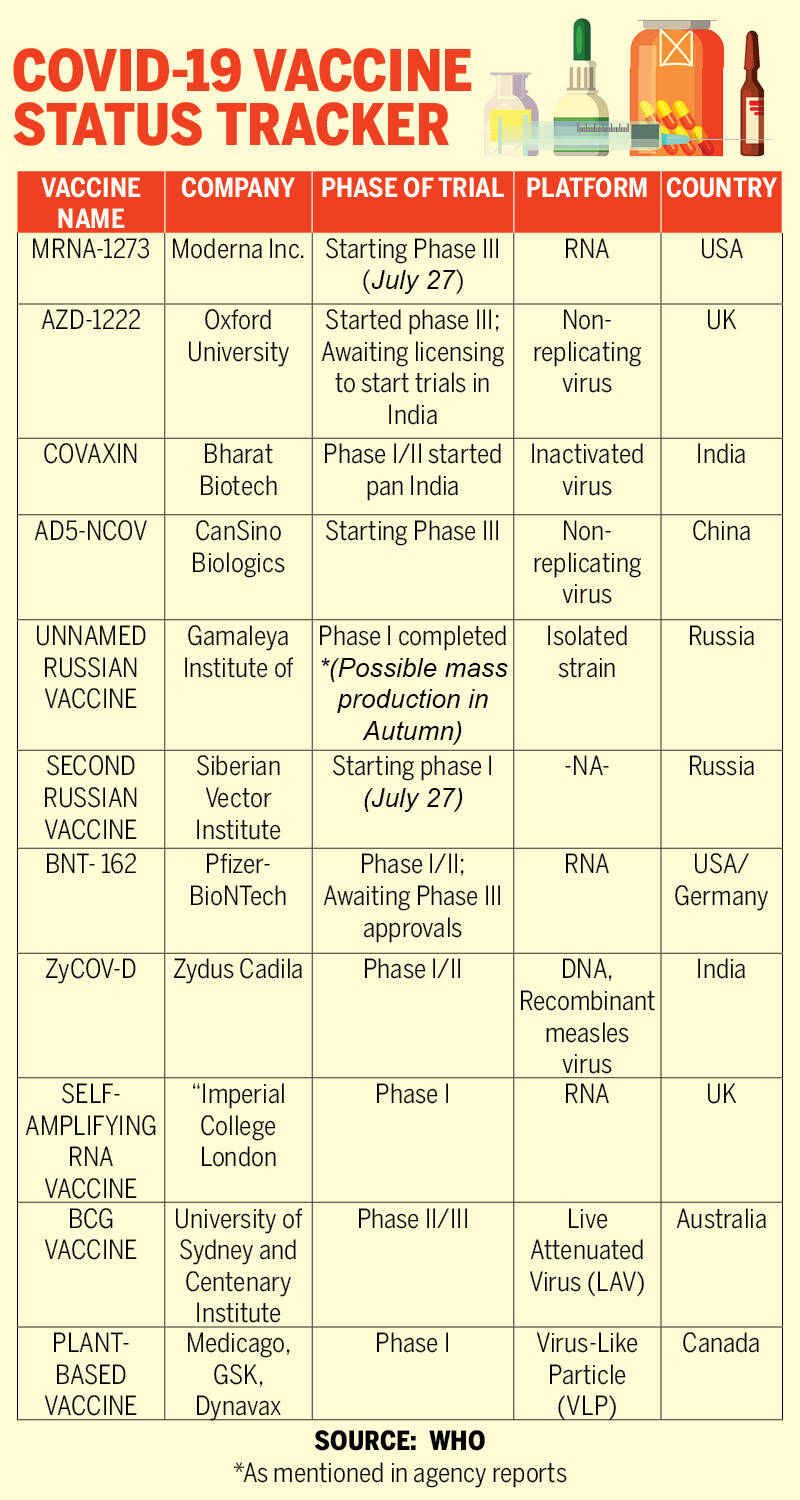
The news comes just three weeks after Bharat Biotech backed Covaxin, which has been developed in collaboration with ICMR and NIV, Pune and Zydus Cadila developed ZyCoV-D was granted licensing to start phase I clinical trials.
While Covaxin was the first Indian vaccine to be issued the go-ahead for the advent of clinical trials in the first week of July, ZyCOV-D was the first vaccine to head to the trials.
The announcement was made by ICMR head Balram Bhargava at a recent press conference. Adding that phase I testing was being completed in several centres across the country, the two vaccines are now undergoing studies for phase II:
“At present, we have three vaccines at different levels of clinical testing. The first one is the inactivated virus vaccine, which is the Bharat Biotech vaccine, which has completed its phase I study in 11 sites and has started its phase II study. Similarly, for the DNA vaccine of Zydus Cadila, India has completed the phase I study and has embarked on phase II studies,”
What does Phase II testing of a vaccine look like?
In stage II of vaccine testing, mid-scale testing takes place. This phase usually involves a larger pool of candidates, which can even run into several hundred who are then split into focus groups of children, healthy adults or elderlies. Depending on the same, different dosing schedules are devised. Several studies are done to study the safety, immuno-response, time is taken to trigger a reaction or side-effects if any.
This is also often referred to as a critical stage of vaccine development since makers usually study the actual safety and other protocols pertaining to vaccine delivery in this mid-scale trial. A lot of vaccine groups, amongst the 110+ candidates are in this phase of testing.
The completion of phase I of both Covaxin and ZyCOV-D has been termed to be faster than Oxford-AstraZeneca vaccine trials, which is currently, one of the most advanced and promising vaccines in the race. Oxford University is also said to start simultaneous phase II/III testing in centres across India, in partnership with Pune based Serum Institute of India (SII).
What next?
Phase II, which will test immunogenicity and safety response of the experimental vaccine will be followed by the most crucial and advanced phase III testing.
Interestingly, authorities had earlier stipulated August 15 as an ambitious launch date for Covaxin.
The news of the launch of phase II of Indian vaccines come after authorities recruited participants for early stages of testing. While both Bharat Biotech and Zydus Cadila have not commented on the announcement yet, early reports suggested that the vaccines have been showing promising results, so far.
Testing conducted at a medical centre in Rohtak, where volunteers have been administrated with doses of COVAXIN showed a good immune response, which was what scientists were hopeful for.
[ad_2]
Source link