
[ad_1]
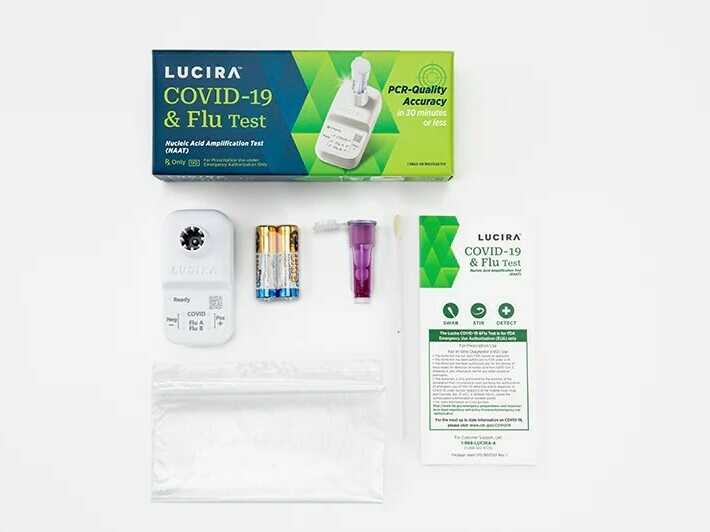
Lucira Health, which developed the dual-purposed diagnostic check, mentioned it might probably present a optimistic consequence as quick as 11 minutes and a destructive end in about half-hour.
Lucira Health
disguise caption
toggle caption
Lucira Health
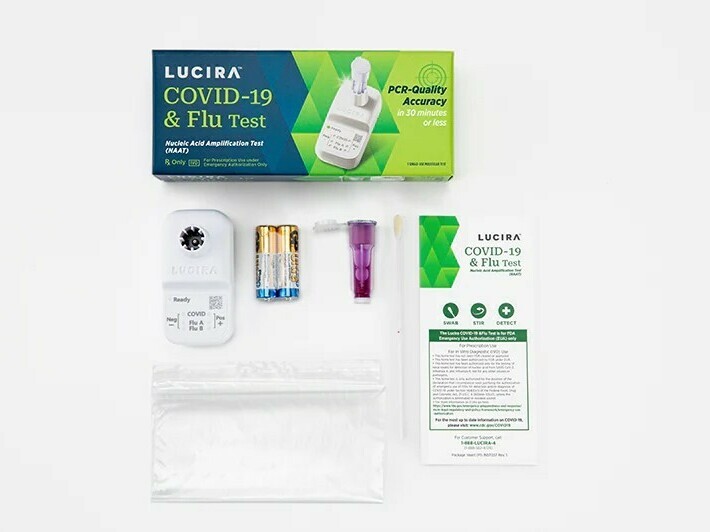
Lucira Health, which developed the dual-purposed diagnostic check, mentioned it might probably present a optimistic consequence as quick as 11 minutes and a destructive end in about half-hour.
Lucira Health
The Food and Drug Administration issued an emergency use authorization on Friday for the primary at-home check that may concurrently detect each COVID-19 and the flu.
With a shallow nasal swab, the single-use package can present outcomes inside half-hour indicating whether or not an individual is optimistic or destructive for COVID, in addition to influenza A and influenza B, that are two frequent strains of the flu.
People 14 and older can typically carry out the check on themselves, the FDA says. Those between the ages of two and 13 can get outcomes with the assistance of an grownup.
Dr. Jeff Shuren, the director of the FDA’s Center for Devices and Radiological Health, referred to as the check as a “major milestone.”
“We are eager to continue advancing greater access to at-home infectious disease testing to best support public health needs,” Shuren said in a press release.
The check was developed by Lucira Health, a California-based firm that was additionally the primary to obtain FDA approval for at-home speedy COVID exams again in 2020.
According to the FDA, in folks displaying signs, the Lucira residence package precisely detected 88.3% of COVID infections and 90.1% of influenza A infections. The check can determine influenza B in lab research, the FDA mentioned. But as a result of there should not sufficient instances of the virus circulating in real-world settings, additional testing might be required, officers mentioned.
The FDA additionally warned that, much like all speedy diagnostic exams, there’s a threat of false optimistic and false destructive outcomes. The company says people who check optimistic for COVID or the flu ought to take acceptable precautions and follow-up with a well being care supplier, whereas individuals who obtain a destructive results of both COVID or influenza B ought to affirm it with a molecular check preformed in a lab.
Individuals who check destructive however proceed to expertise signs of fever, cough or shortness of breath also needs to comply with up with their well being care supplier in case of different respiratory viruses, the FDA mentioned.
The dual-purposed check comes after a surge of COVID, the flu and respiratory syncytial virus — or RSV — that strained hospitals throughout the nation final fall.
“The collective impact of COVID-19, flu and RSV underscore the importance of diagnostic tests for respiratory viruses,” the FDA mentioned in a press release.
Over the previous few weeks, COVID-related deaths and hospitalizations have begun to fall, in accordance with the most recent information from the Centers for Disease Control and Prevention. Similarly, charges of flu and RSV-related hospitalizations have been taking place, the CDC found.
[adinserter block=”4″]
[ad_2]
Source link


