.jpg)
[ad_1]
Accurate knowledge of both current and previous coronavirus disease 19 (COVID-19) cases is essential to control the rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Serological assays can help identify individuals previously exposed to the virus who may potentially be immune. In addition, cell-based serological screening platforms can aid in identifying antibodies that may be undetectable by other methods deployed to study the antigenicity of the SARS-CoV-2 proteome.
A new study published on the medRxiv* preprint server used automated high content immunofluorescence microscopy (AHCIM) to assess the viability of such an approach to detect humoral immune responses against SARS-CoV-2 proteins.
Motivation
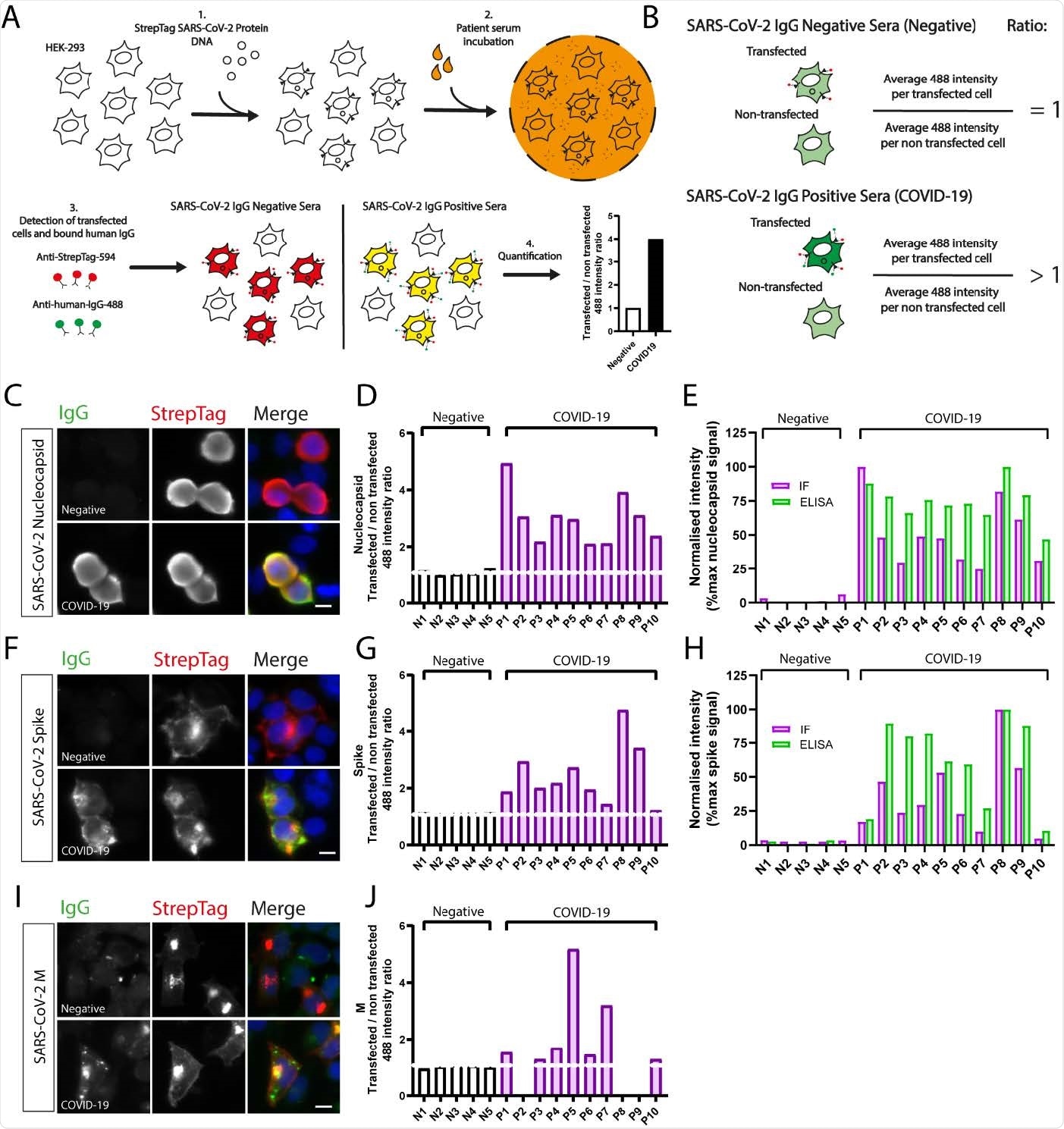
The baseline aim of this study was to understand the usefulness of immunofluorescence-based serological screening platforms to identify nucleocapsid (N) and spike (S) antibodies in SARS-CoV-2 infected individuals.
The applied method is widely used by countless laboratories worldwide for many purposes. Therefore, scientists hypothesized that this method could be used for various purposes related to SARS-CoV-2 serology if of acceptable sensitivity and specificity.
The Study
To date, no study using a high throughput immunofluorescence microscopy-based system has thoroughly examined antibody responses in human serum samples against SARS-CoV-2 proteins.
Scientists found that automated high content immunofluorescence microscopy (AHCIM) was efficient in quickly screening sera for antibodies against the SARS-CoV-2 N, S, and M (membrane) proteins.
Using a semi-quantitative method, they generated IgG ratios from mean fluorescence intensity values per non-transfected and transfected cell. High IgG ratios indicated strong antibody responses. Subsequently, the ratios were used to assess the sensitivity and specificity of AHCIM to detect N and S IgG. It was observed that the results were equivalent to those seen for absorbances measured by ELISA.
Decreasing the number of transfected cells improves 488-intensity ratios. (A) Representative images of StrepTagged SARS-CoV-2 nucleocapsid transfected cells imaged at different ratios of transfected to non-transfected cells. Serum samples with strong (A09), intermediate (C01) and weak (C08) SARS-CoV-2 nucleocapsid IgG responses were selected. Transfected cells plated at 100% confluency were split at the indicated dilutions, fixed, incubated with patient sera and processed for immunofluorescence as described. (B) Automated quantification of 488 intensity ratios for COVID19 serum samples A09, C01 and C08 and the COVID19 negative serum sample D06. Decreasing cell density increases the IgG ratio for strong, intermediate and weak COVID-19 positive samples.
Scientists noted that ELISA-based methods require access to purified proteins, and in case these are not available, AHCIM may be a cheaper and faster alternative to detect SARS-CoV-2 N and S antibodies.
Previous studies mainly reported low throughput qualitative assessment of antibody titers. The current study presents a fast, high throughput, and semi-quantitative method and is, therefore, an upgrade over previous serological screening methods.
Once the system of detecting N and S proteins was validated, researchers progressed on to investigating antibody responses to the SARS-CoV-2 M protein.
Previous studies had identified detectable antibody responses against the SARS-CoV M protein, which served as the motivation for this study.
Researchers observed that 84.7% of COVID samples tested had detectable IgG against M. These results have been corroborated by another recent study that also found a high prevalence of M antibodies in COVID-19 positive patient sera, using flow cytometry.
Compared to other studies, a high number of COVID-19 patients with antibodies to M were detected in this study. This could be driven by the state in which viral proteins are assayed for reactivity with patient sera. Generally, a high seroprevalence of M antibodies has been observed in COVID-19 patients. Therefore, M antibodies screening may be valuable to SARS-CoV-2 serological testing.
Several complications in antibody testing arise from the widespread use of the S protein as the immunogen used in vaccines. This underscores the utility of the M protein.
Previously, vaccinations combining S and N as immunogens have also been proposed, as that would reduce the need for both N and S antibody tests. This further highlights the significant potential role of M as a serological marker.
To prove this, scientists identified cases classified as COVID-19 negative (owing to the lack of detectable N antibodies) but were reclassified as positive through screening for M antibodies by AHCIM.
They opined that complementing ELISA with AHCIM could enhance the sensitivity and specificity of SARS-CoV2 serological testing. Furthermore, long-lived serological markers enable optimal COVID-19 serosurveillance. Preliminary analysis of N and M IgG ratios (plotted against days post PCR test) showed a declining trend in N over time, but not for M.
Future Research
While preliminary results are encouraging, more research on N and M antibody titers is required to establish M as a long-lived serological marker compared to N.
Other important questions are related to the correlations between the presence of M antibodies and disease outcomes and if antibodies against M bolster the neutralizing activity of humoral immune responses generated against SARS-CoV-2.
Addressing these research questions could aid immensely in vaccine development and guide which antigens could be used as markers of prior infection in diagnostic methods.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
[ad_2]
Source link
.jpg)