
[ad_1]
Vanderbilt researchers awarded one of NSF’s 24 new projects to drive future manufacturing
One of the challenges of drug delivery systems is to optimize their targeting properties so therapeutic compounds used in smaller amounts reach only a specific area of the body and result in little or no side effects.
The ability to engineer the content of extracellular vesicles and target these EVs to specific sites offers the potential to develop a new class of cellular therapeutics that have “exquisite targeting ability” and avoid the side effects and toxicity associated with traditional drug delivery approaches.
EVs are cellular communicators—particles that are naturally secreted and carry a selectively packaged cargo of proteins, nucleic acids, lipids and more from a parent cell to a recipient cell—but do not replicate, unlike cells. EVs are now considered leading actors of intercellular communication, mediating both physiological as well as pathological responses. EV therapeutics are being developed for treatment of a wide range of diseases including diabetes, dementia, heart ailments and cancer.
An interdisciplinary team of Vanderbilt researchers led by Jamey Young, professor of chemical and biomolecular engineering, has received a two-year, $500,000 Future Manufacturing Seed Grant for their work—Enabling Technologies for Biomanufacturing EV-Based Therapeutics—from the U.S. National Science Foundation.
The NSF today announced investments in 24 new projects to drive future manufacturing. The program has awarded more than $40 million in the form of seed grants, research grants and network grants that will support research and education at 44 unique institutions in 18 states and the District of Columbia.
One of 14 NSF seed grants, the Vanderbilt researchers’ long-term goal is to develop technologies for production of “designer EVs” that can be packaged with desired cargo molecules, decorated with tunable surface ligands, and secreted in high yield from specific producer cell types. These technologies for future EV biomanufacturing are expected to promote the growth of a new class of pharmaceutical products.
“Our investment provides industry with manufacturing tools that currently live only in the laboratory, or the imagination,” said NSF Director Sethuraman Panchanathan. “Through the convergence of such fields as robotics, artificial intelligence, biotechnology, and materials research, future manufacturing will create revolutionary products with unprecedented capabilities, produced sustainably in facilities across the country by a diverse, newly trained workforce.”
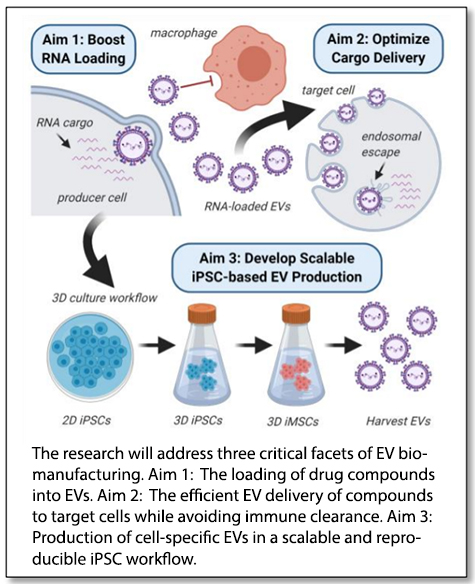 “We will focus our research on loading and delivery of small RNAs that have shown tremendous promise for modulating previously ‘undruggable’ targets but are not efficiently delivered to recipient cells using established nanoparticle-based carriers,” Young said. Senior team members include Ethan Lippmann and John Wilson, assistant professors of chemical and biomolecular engineering, and physician-scientist Alissa Weaver, Cornelius Vanderbilt Professor of Cell and Developmental Biology in the School of Medicine.
“We will focus our research on loading and delivery of small RNAs that have shown tremendous promise for modulating previously ‘undruggable’ targets but are not efficiently delivered to recipient cells using established nanoparticle-based carriers,” Young said. Senior team members include Ethan Lippmann and John Wilson, assistant professors of chemical and biomolecular engineering, and physician-scientist Alissa Weaver, Cornelius Vanderbilt Professor of Cell and Developmental Biology in the School of Medicine.
Ribonucleic acid, or RNA, is a nucleic acid present in all living cells and acts as a messenger carrying instructions from DNA for controlling the synthesis of proteins. Small RNAs are short RNA molecules that can regulate gene expression. Increasing evidence indicates that small RNAs are involved in the development of diverse diseases including cancer, cardiovascular disease, stroke, neurodegenerative disease, diabetes, liver disease, kidney disease and infectious disease. Multiple studies have shown that small RNAs may serve as novel biomarkers and therapeutic targets for many diseases.
“RNA and many other biologic therapeutics, like enzymes, proteins, and DNA, have immense and still almost entirely untapped potential, but critical physiological barriers prevent these molecules from reaching the correct cell type at high enough amounts while sparing healthy tissue,” Wilson said.
Vanderbilt University has significant expertise in EV molecular biology related to Weaver’s extensive research, Wilson’s work on nanoparticle-mediated drug delivery, Lippmann’s stem cell culture and differentiation research, and Young’s expertise in cell-based biomanufacturing. The grant will promote expansion of an EV-based research hub at Vanderbilt, which also will lead to the opportunity to train a technical workforce to support the emerging EV biotherapeutics industry, Young said.
“EVs are our natural, endogenous drug delivery systems and are capable of packaging and shipping diverse cargo all throughout the body. We believe that if we can better understand the remarkable capacity of EVs to do this and then also engineer them with enhanced functionalities that further optimize their performance, then this approach has potential to transform the treatment of a wide range of important diseases,” Wilson said.
“Our team has developed cutting-edge molecular biology tools for evaluating the efficiency of RNA delivery from EVs to target cells. The Weaver lab has significant expertise in genetic engineering of EV-producing cells, biochemical analysis of EVs, and design of reporter cell systems for evaluating mechanisms of EV synthesis and RNA delivery,” Young said.
“This project is exciting because it brings together such complementary expertise in EV cell biology, drug delivery, and biomanufacturing processes. In addition, this project will bring a new focus on therapeutics to the Vanderbilt EV Community. Importantly, by taking an engineering approach this project will address big questions in the field in a new way, including how to boost therapeutic EV cargoes and improve the efficiency of their functional delivery to recipient cells,” Weaver said.
The team will focus on production of EVs from human induced pluripotent stem cell (iPSC)-derived mesenchymal stem cells (MSCs). MSCs are derived from human tissues such as bone marrow, body fat, and placental tissue and are widely clinically used as therapeutic agents for tissue repair and regeneration. Human iPSCs overcome challenges presented by MSCs. Importantly, they can be expanded in culture indefinitely while maintaining full developmental potential.
“Although we have proposed to study the production of EVs from one type of cell, the MSC, this strategy will establish our approach and potentially allow us to extend into production of EVs from other types of cells with different therapeutic potential,” Lippmann said. “The power of iPSCs is that they can theoretically be differentiated into any cell type in the human body, and we could then harvest EVs from those cells for specific applications.”
The team will address three major knowledge gaps that pose barriers to the development of EV-based therapeutics:
- How does cargo get loaded into EVs? Cells naturally select cargo—proteins, nucleic acids, lipids—that is packaged into EVs and delivered to other cells. The researchers want to know how to control the loading of specific molecules, which could be selected as a therapeutic cargo.
- How can we control where the EVs go in the body? This is the targeting ability the team will address—how to get the EVs to the desired location before they are cleared by the immune system.
- What is the best way to produce therapeutic EVs? The team will examine and evaluate the best ways to scale up iPSC differentiation and cultivation workflows for EV manufacturing.
The purpose of the seed grants is to support fundamental, early-stage research in thrust areas like biomanufacturing that have the potential for clinical impacts and commercialization related to new manufacturing opportunities for established companies and for entrepreneurs. Awards in the seed grant track provide support for up to two years at a level up to $250,000 each year. It is anticipated that successful outcomes from research supported by seed grants will be eligible for NSF Future Manufacturing Research Grants, which range from $500,000 to $2 million per year for up to five years.
“Vanderbilt already is a leader in studying the role of EVs in cancer and other diseases. Establishing a research hub at Vanderbilt to explore the basic and applied science of cell-based EV production will accelerate new technologies that can have immediate commercial and clinical impacts,” Young said.
This research is supported by the National Science Foundation, award number 2036809.
Contact: Brenda Ellis, 615 343-6314
brenda.ellis@vanderbilt.edu
Posted on Friday, October 2, 2020 in Alissa Weaver, biomanufacturing, Ethan Lippmann, EVs, extracellular vesicles, grant, Jamey Young, John Wilson, NSF,Alumni, Chemical and Biomolecular Engineering, Home Features, Media, News, News Sidebar, Research
[ad_2]
Source link
