
[ad_1]
Nanoporous membranes have been proven to be invaluable instruments for filtering out impurities from water and quite a few different purposes. However, there’s nonetheless a lot work to be carried out in perfecting their designs. Recently, the lab of Prof. Amir Haji-Akbari has demonstrated that precisely the place the nanosized holes are positioned on the membrane could make a giant distinction. The outcomes are in ACS Nano.
In latest years, nanoporous membranes comprised of graphene, polymers, silicon and different supplies have been used efficiently for separating fuel, desalinating water, virus filtration, energy era, fuel storage, and drug supply. However, creating membranes that allow all the fitting molecules cross by means of whereas retaining the undesired ones out has confirmed difficult.
For desalinating water, as an illustration, it’s essential that the membrane has a excessive permeability for water whereas sufficiently blocking small ionic and molecular solutes, and different impurities. But researchers have discovered that enhancing the permeability of a membrane usually compromises its selectivity, and vice versa.
One promising method is to optimize the chemistry and geometry of remoted nanopores to attain the specified permeability and selectivity, and place as a lot of these pores as attainable inside a nanoporous membrane. Exactly how neighboring pores have an effect on one another, although, is unclear. At the nanoscale, molecules interacting with pore partitions can exhibit behaviors that defy standard theories. The Haji-Akbari lab explored whether or not they may design progressive membrane programs with elevated precision and effectivity by fine-tuning the nanopores.
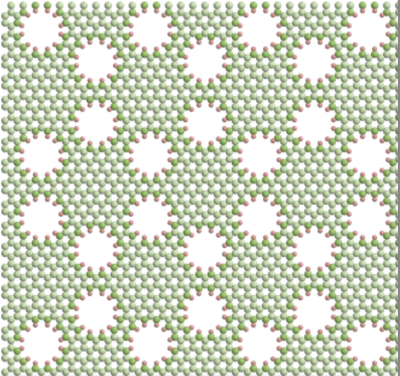 With laptop simulations, Haji-Akbari’s analysis staff discovered that nanoscale proximity between pores can detrimentally have an effect on water permeability and salt rejection. Specifically, they created simulations of membranes with various patterns of pore placement, together with a hexagonal lattice (determine at left) and a honeycomb lattice (proper). What they discovered was that the hexagonal sample, which allowed for extra distance between pores, had a larger permeability/selectivity efficiency than the membrane with the honeycomb sample.
With laptop simulations, Haji-Akbari’s analysis staff discovered that nanoscale proximity between pores can detrimentally have an effect on water permeability and salt rejection. Specifically, they created simulations of membranes with various patterns of pore placement, together with a hexagonal lattice (determine at left) and a honeycomb lattice (proper). What they discovered was that the hexagonal sample, which allowed for extra distance between pores, had a larger permeability/selectivity efficiency than the membrane with the honeycomb sample.
These results deviate from established theories, Haji-Akbari stated.
“This assumption that the pore resistance is independent of the proximity of the pore is not correct,” stated Haji-Akbari, assistant professor of chemical & environmental engineering. “Clearly, it depends on proximity.”
Their findings shed perception on how these results speed up the actions of sure ions by means of membranes whereas inflicting different ions to decelerate. Further, it may well inform higher designs of nanoporous membranes for enhanced separation processes akin to water desalination and different purposes.
[adinserter block=”4″]
[ad_2]
Source link