
[ad_1]
Written by ANURADHA MASCAREHNAS | Pune |
Updated: August 27, 2020 8:40:26 am
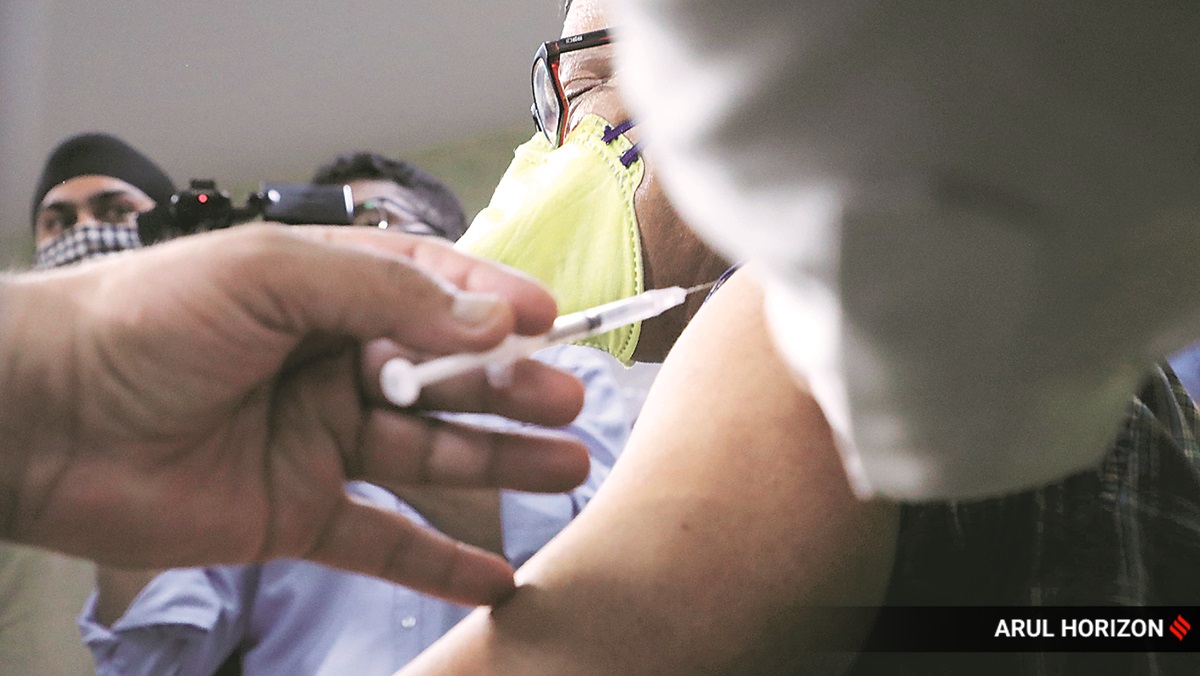 Covishield, the vaccine candidate, being administered to a volunteer in Pune. (Express photo: Arul Horizon)
Covishield, the vaccine candidate, being administered to a volunteer in Pune. (Express photo: Arul Horizon)
Two male volunteers at Pune’s Bharati Hospital became the first to be injected as phase 2/3 clinical trials of the Oxford-AstraZeneca Covid-19 vaccine candidate began in India on Wednesday.
One of the volunteers was a 48-year-old gynaecologist at a private hospital in Pune who had also volunteered 10 years ago for clinical trials for a vaccine against the H1N1 (swine flu) virus.
The other — the first volunteer to be injected with the candidate that has been named Covishield in India — is a 32-year-old doctoral candidate in statistics who works for a private company in the city.
A total 1,600 volunteers will be part of the study at multiple trial sites. The Drug Controller General of India (DCGI), the national drug regulator, had on August 3 allowed Pune-based Serum Institute of India, the world’s largest manufacturer of vaccines, to conduct phase 2/3 human clinical trials of the vaccine candidate in India.
The vaccine candidate, ChAdOx1 nCoV-19 or AZD1222, is already undergoing advanced human clinical trials in Brazil, South Africa, and the United Kingdom. Oxford University researchers announced last month that the candidate had triggered a dual immune response in phase 1 and phase 2 human clinical trials against the coronavirus that causes Covid-19, and had shown an “accepted safety profile”.
“My (then) 11-year-old daughter participated in a clinical trial during the H1N1 pandemic and, 10 years later, we have enlisted our support as volunteers for the Covishield vaccine trial,” the 48-year-old gynaecologist told The Indian Express.
The 32-year-old, who belongs to a family of farmers, and who studied in Aurangabad, said: “Participating in the trial is my contribution to beating the virus that has claimed millions of lives globally.”
At a well-attended media event, the 32-year-old received his shot at 1.35 pm. The second volunteer was injected 15 minutes later, at 1.50 pm.
“Five volunteers were screened on Tuesday and RT-PCR and antibody tests were conducted. Three volunteers tested positive for antibodies against Covid-19, which means they may have had the infection at some point, and they cannot, therefore, participate in the trial,” Dr Sanjay Lalwani, Medical Director of Bharati Vidyapeeth Medical College and Hospital, said.
Most clinical trial sites have seen a rush of volunteers for the study. Each of the four sites in Pune has seen 250-300 volunteers. Among those who enrolled on Wednesday evening for screening at Bharati Hospital were the 48-year-old gynaecologist’s 47-year-old wife and their daughter, who is now a 22-year-old student of engineering.
The CEO of a well known firm and his family, along with their driver, were also screened, hospital authorities said.
The gynaecologist said he had seen several doctors and healthcare workers getting infected with the virus, and was feeling good about being part of the trial for the vaccine.
“I am not feeling excited, rather good about taking part in this trial as I have seen a few die due to complications related to the disease. Science is the only way to fight this virus,” he said.
“I am aware that I may have got a placebo instead of the vaccine, and I am mentally prepared for that. I have even seen a lot of people trying to sell immunity-boosters in the time of the pandemic. This is not correct, and I strongly feel that a vaccine can be the solution.”
The gynaecologist said his wife and daughter would get their shots on Thursday if they cleared the tests for the virus and antibodies. It would be the first clinical trial for his wife and the third for their daughter, he said. “She (the daughter) has earlier participated in a trial for a vaccine for cervical cancer.”
The vaccine candidate has been developed by the Jenner Institute at Oxford University from a weakened version of a common cold virus (known as adenovirus) from chimpanzees that has been modified so it cannot grow in humans. Serum Institute has tied up with British-Swedish pharmaceutical firm AstraZeneca to produce a billion doses of the vaccine for low-income countries.
Dr Asmita Jagtap, executive director of Bharati Vidyapeeth’s health sciences section, said the institution had been involved in over 50 clinical trials over the last 15 years. “We have been getting several calls from people wanting to enlist as volunteers,” she said.
Dr Jitendra Oswal, deputy medical director of Bharati Hospital, said 100 volunteers will participate at some sites. “We will enlist 25 trial participants at our site,” he said.
On Wednesday, KEM Hospital and Research Centre at Vadu, some 20 km from Pune, too began screening volunteers. Five persons will be administered shots on Thursday at this trial site, provided they test negative for Covid-19.
Jehangir Hospital and Sassoon General Hospital, the other two trial sites in Pune, were still waiting for their doses of the candidate on Wednesday.
Optimism and excitement are running high at the trial sites – at Bharati Hospital, security officer Mallika Sache said: “India phir se aazaad ho jayega (The vaccine will liberate India again).”
📣 The Indian Express is now on Telegram. Click here to join our channel (@indianexpress) and stay updated with the latest headlines
For all the latest India News, download Indian Express App.
© The Indian Express (P) Ltd
[ad_2]
Source link