[ad_1]
It began as a “regular”, low-grade fever towards the end of July. Like on similar occasions in the past, Alieu Kijera took his son, Mohammed Lamin Kijera, to the nearest health centre where he was prescribed a syrup and some medicines. By 5 pm, the child’s condition worsened. Days later, on August 4, Lamin – 2 years and 5 months old – passed away.
What followed over the next few days upended Kijera’s life – thrusting him and his family to the centre of one of the worst medical tragedies witnessed in The Gambia, a tiny country on the western coast of Africa. Lamin was among 69 children who died in The Gambia with acute kidney injury, with the World Health Organisation “potentially” linking their deaths to contaminated cough syrups manufactured in India. All the four syrups that are suspected to have led to the deaths — Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup – were manufactured by the Haryana-based Maiden Pharmaceuticals.
Speaking to The Indian Express on video from his home in the Gambian town of Latrikunda, Kijera, a nurse who works at a local hospital, says he is still to come to terms with the rapid slide in events that ended in his son’s death. After that first visit to the health centre, where Lamin was prescribed the syrup and some medicines, his condition worsened by 5 pm the same day. “So I took him back to the health centre, where they put him on drips. He started feeling better and I brought him home,” says Kijera, adding that Lamin was “okay” over the next few days until, one day, he stopped passing urine. Kijera took Lamin back to the health centre, where he was asked to undergo a kidney test.
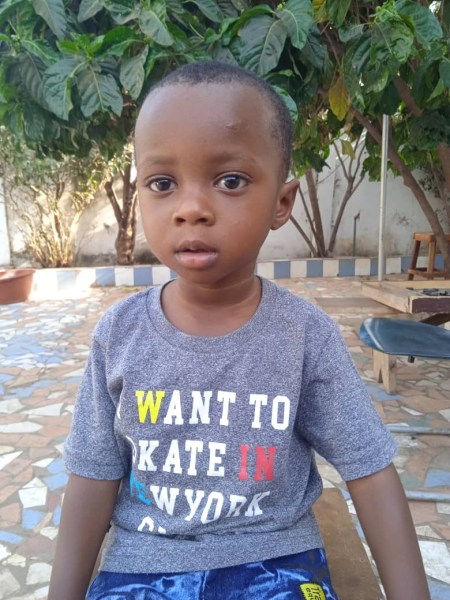 Mohammed Lamin Kijera, 2 years, died in August
Mohammed Lamin Kijera, 2 years, died in August
The results confirmed the worst. “The doctors said Lamin’s kidneys were damaged and he needed dialysis,” says Kijera, adding that the child was referred to the country’s biggest hospital – the Edward Francis Small Teaching Hospital – in Gambia’s capital of Banjul.
But with the child’s condition deteriorating, the doctors referred Lamin to a hospital in Dakar, the capital of neighbouring Senegal. There, the child underwent an emergency dialysis procedure, but the end came a week later.
“My son was among the first five cases of acute kidney injury reported in the country. The doctors did not know what was happening at the time. Three of four other children who had been referred to the same Banjul hospital as Lamin passed away in front of us,” says Kijera, sitting on a brown sofa in his house. Behind him, on one of the walls of his living room, are photographs of Lamin with his two elder sisters.
Around 4 km away are the Kamasos, united in their grief with the Kijeras – on September 1, the family lost their youngest member, Musa, one year and seven months old.
 Musa Kamaso, 1 year, died in September
Musa Kamaso, 1 year, died in September
By the time of Musa’s death, however, hospitals in The Gambia were aware of an increase in cases of acute kidney injury among children – and looking at the possibility that these cases could be linked to syrups prescribed for fever, cough and cold.
Musa had come down with fever by August-end. When he was taken to the local health centre, the doctors ruled out malaria. “They prescribed him medicines that were not available at the health centre. I bought them from a private pharmacy,” says Kamaso. Four days later, the child stopped passing urine.
“He also had diarrhoea and was vomiting. So we took him back to the health centre on Saturday morning. This time, they referred him to a bigger hospital. But there, too, the doctors were not able to figure out what was wrong,” says Kamaso, who runs a small business in Tallinding, a suburb of Banjul.
Musa was finally admitted to the Edward Francis Small Teaching Hospital, around half an hour from their home.
A lab test revealed that the child had acute kidney failure. “I immediately called my wife to ask if he had fallen down somewhere… How can a baby have this kind of disease? The doctors put Musa on dialysis but his condition worsened. I called his mother and told her to prepare for the worst,” says Kamaso, speaking from his tin-roofed house in Tallinding. In the background is a red bike, “Musa’s favourite”.
Musa, the youngest of Kamaso’s five children – four sons and a daughter — passed away early morning on September 1.
The parents of Lamin, Musa and the other victims have now formed a WhatsApp group and plan to file a case against the Haryana-based company.
Angry and bitter at how his life has unravelled, Kamaso says, “I learned about the problem from inspectors who came to ask us about Musa’s death. How can a company sell contaminated drugs? They should have tested it properly before releasing it to the market. This is murder!”
In The Gambia, a recall, fear
The day the WHO linked the deaths of children to the allegedly contaminated syrups, The Gambia started a five-day, door-to-door campaign to recall the syrups.
The Haryana-based company, Maiden Pharmaceuticals, had exported 50,000 bottles of fever, cough and cold syrups to The Gambia on a purchase order. At the end of the drive, the Gambian authorities had collected 41,462 bottles, with 8, 538 bottles remaining unaccounted for.
In a press conference earlier this month, The Gambian Health Minister Dr. Ahmadou Lamin Samateh said 81 cases of acute kidney injury were reported in the country. With 69 deaths, the case fatality ratio stood at 85%. “We are no longer seeing many cases and that is a good sign,” he said in a press conference, adding, “We as a country are a victim of this problem, these contaminated drugs were not manufactured here.”
Biram S Jobe, environmentalist and media practitioner in The Gambia, said the incident has led to a lot of fear in the country. “Many people do not want to go to government hospitals now for fear that they might be given these medicines,” he said.
More importantly, he said, The Gambia does not have a drug quality testing laboratory and the World Bank was helping it set one up so that such incidents do not recur. The Indian Health Ministry had in a statement said that it is usual practice for the importing country to test drugs before releasing them in the market.
In India, action against manufacturer
After being alerted by the WHO, India’s apex drug regulator, the Central Drugs Standard Control Organisation, and Haryana’s state drug controller initiated a joint investigation and found some glaring gaps in the cough syrups manufactured by Maiden Pharmaceuticals and exported to The Gambia – among them, the use of a solvent which was set to expire before the expiry date of the medicine; solvent not tested for contaminants; discrepancies in manufacturing dates; and batch numbers missing from key test report.
On Wednesday, the Haryana drug control department ordered a “complete stop” on manufacturing activity at the company’s factory in Sonepat.
Meanwhile, control samples – samples from the batches that were exported, stored by the company as part of quality control process – have been sent for testing at the regional drug testing laboratory in Chandigarh. Officials said the company will face further action depending on these reports.
The Indian government has also asked the WHO to send documentation to establish a causal link between the deaths and the syrups. A four-member committee was also set up to review these documents.
[adinserter block=”4″]
[ad_2]
Source link