.jpg)
[ad_1]
The rapid spread of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in the coronavirus disease 2019 (COVID-19) pandemic that has infected around 245 million individuals and claimed more than 4.97 million lives worldwide. The SARS-CoV-2 virus is a positive-sense RNA virus belonging to the family Coronaviridae of the genus Betacoronavirus. This virus contains four structural proteins, namely, spike, envelope, membrane, and nucleocapsid.
Anti-nucleocapsid (N) antibody and COVID-19 Infection
A new study, published on the medRxiv* preprint server, has focused on the use of anti-nucleocapsid (N) antibody positivity as a marker of SARS-CoV-2 infection. In the present study, these antibody markers were evaluated using serological surveillance studies post-vaccination amidst circulation of variants of concern.
A previous study included twenty-three participants who experienced a breakthrough infection about fourteen days after they were vaccinated with the second dose of the vaccine. Among this study cohort, six showed detectable N antibodies 9-67 days post-infection. Considering the low sensitivity of N assays, this study had a strong influence on the interpretation of the serosurveillance studies of highly vaccinated populations.
The present study evaluated the seropositivity results of convalescent N antibody in both vaccinated and unvaccinated individuals after Alpha or Delta variant infections. This study obtained data from the Public Health England who collected random samples of vaccine-eligible individuals identified from community testing data. All the individuals identified tested positive for COVID-19. This data was collected from February 2021. Scientists determined the variants of each infection by sequencing or as per the date of PCR test, i.e., if the PCR date was before 03/05/2021, the strain was assumed to be Alpha, and if the PCR date was after 24/05/2021, the strain was considered to be the Delta.
This study obtained convalescent N antibody results for 472 PCR positive cases recruited for the study. Researchers observed that in the study cohort, at the time of infection, 143 individuals were unvaccinated, and 176 had received a single dose of the vaccine, and infection occurred 21 days after the vaccination. Eighty participants showed symptoms after seven days of receiving the second dose of the vaccine.
Scientists included convalescent data which was a mixture of various samples. For instance, samples from individuals infected by the original SARS-CoV-2 strain in the past were collected a maximum of 431 days post-infection. Recent infections were mostly by Alpha or Delta variant, across wide-ranging ages. Researchers used two panels of pre-COVID baseline sera, i.e., 472 residual from hospitals and laboratories and 995 from the Oxford biobank. 91% of sera samples (9 months post-infection) were found to be seropositive. Researchers stated that the waning of antibodies over time led to reduced sensitivity.
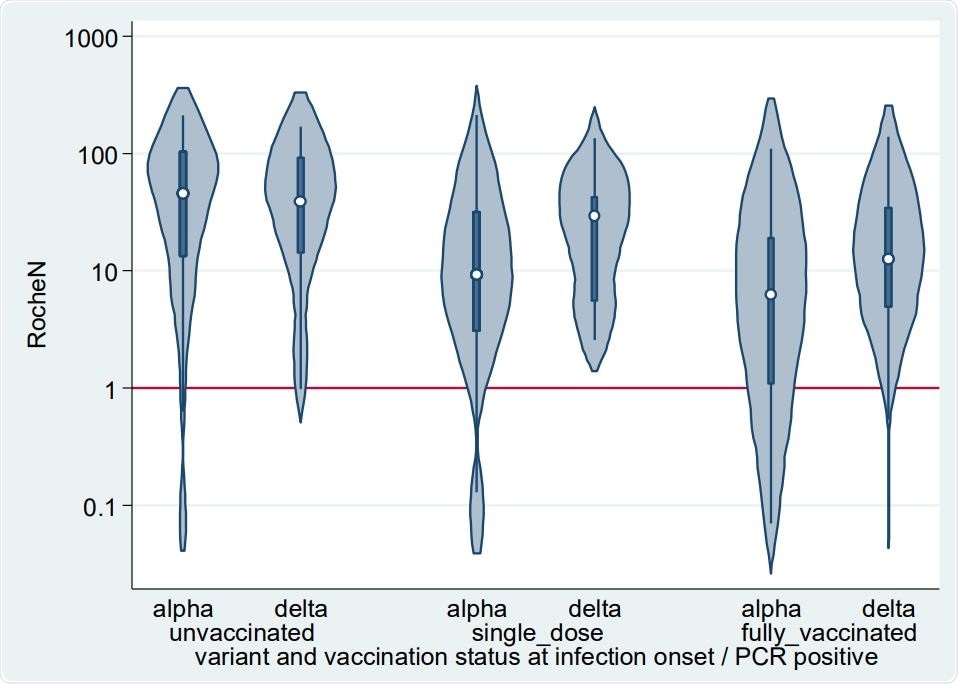
Violin plots depicting the smoothed distribution of Roche N levels (AU/ml) from convalescent sera. The white spot shows the median and the darker box the 25th and 75th percentiles. The horizontal red line is the assay positive/negative cut-off at 1.
The authors of the present research stated that levels of N-antibody were found to be lower following vaccination and differed across variants. Its concentration was generally higher after a Delta infection, and seroconversion rates remained unchanged by vaccination status at the time of infection. After Alpha infection, The N-antibody levels were generally lower, and 78% of fully vaccinated individuals were seroconverted. This was consistently higher than the 26% that was reported in the previous study. Scientists stated that some of the sera samples were possibly collected too soon to observe seroconversion and 18 PCR positive individuals were asymptomatic. This finding indicated low seroconversion following very mild infections.
All Delta infections in the study cohort were symptomatic in this study, while 20 of 279 Alpha infections were asymptomatic. Researchers revealed among these asymptomatic Alpha infections, 65% of samples showed N seroconversion. In the case of sera of symptomatic Alpha infection, 91% of samples indicated N conversion. Among the sera samples that did not seroconvert, approximately 27% N levels were just below the threshold for positivity.
Determination of Optimal Assay Threshold Levels for Serosurveillance
A previous study fixed the Roche N assay cut-off to be 1, which offered a clinical specificity of 99.8%. The authors of this study re-evaluated an optimal cut-off of the Roche Elecsys anti-N assay by measuring the specificity, sensitivity, and area under the receiver operating curve (ROC) for a range of values.
The authors of this study recommended an equivocal range for the Roche Elecsys anti-N assay of 0.4-<1, which could be used to improve sensitivity while retaining a high specificity of 99.6%. They further emphasized that the presence of N antibodies serves as a specific marker for previous symptomatic infection regardless of vaccination status.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
[ad_2]
Source link
.jpg)