
[ad_1]

Mariama Kuyateh, 30, holds up an image of her son Musa, whose dying from acute kidney failure on Oct. 10 was linked to contaminated cough syrup imported to Gambia, the place they stay, from India. The World Health Organization issued an alert in regards to the medicine.
Milan Berckmans/AFP by way of Getty Images
conceal caption
toggle caption
Milan Berckmans/AFP by way of Getty Images

Mariama Kuyateh, 30, holds up an image of her son Musa, whose dying from acute kidney failure on Oct. 10 was linked to contaminated cough syrup imported to Gambia, the place they stay, from India. The World Health Organization issued an alert in regards to the medicine.
Milan Berckmans/AFP by way of Getty Images
In December 2022, the World Health Organization linked Indian-made cough syrups to the acute kidney failure and deaths of 66 kids within the West African nation, The Gambia. WHO’s laboratory evaluation stated the cough syrups contained “unacceptable amounts of diethylene glycol and ethylene glycol,” chemical compounds usually meant for industrial use.” WHO called for manufacturers to check the quality of medical products to prevent this from happening. Shortly afterward, Indian authorities shut down Maiden Pharmaceuticals in the Northern Indian state of Haryana, near Delhi, where the medicines were manufactured. At the time, NPR spoke about the issue with Dinesh S. Thakur, a public health activist, and lawyer Prashant Reddy T, authors of The Truth Pill: The Myth of Drug Regulation in India. In this follow-up interview, we examine official responses to this and other cases and check back with the authors for their perspective. Here are five takeaways.
Over the following week, we’ll be trying again at a few of our favourite Goats and Soda tales to see “whatever happened to …”
Indian pharma remains to be an enormous drive.
In spite of the Gambia controversy and different circumstances of allegedly poisonous or ineffective medicine, the trade is flourishing. India’s drug producers account for 20% of the worldwide demand for medication and 60% of vaccines used worldwide. Its largest success story has been within the export of generics. A generic drug has the identical chemical elements as well-known branded medicines however could be offered at a less expensive fee after the patent for the higher-priced branded drug expires. Sales of those generics at present account for 70% of India’s export revenue that it earns from pharma.
“Year on 12 months, the Indian pharma industry has grown and there aren’t any indicators of slowing down,” says Prashant Reddy.
Over the previous 12 months, further made-in-India medication have come underneath hearth.
Since October 2022, the World Health Organization has issued 6 medical alerts for contaminated syrups and estimates that 15 Indian corporations are concerned of their distribution. Investigations with India’s Central Drugs Standard Control Organisation are ongoing.

These cough syrups have been collected in Banjul, the capital of Ghana, on October 6, 2022.
Milan Berckmans/AFP by way of Getty Images
conceal caption
toggle caption
Milan Berckmans/AFP by way of Getty Images
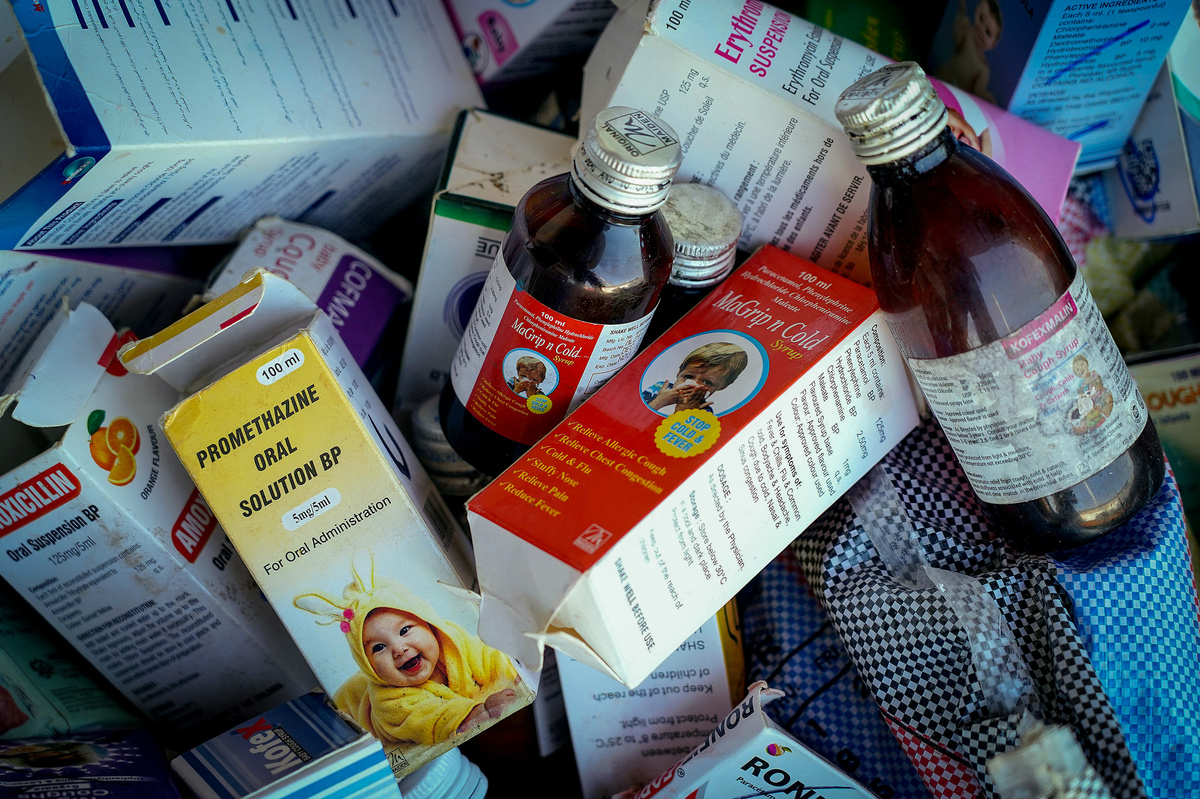
These cough syrups have been collected in Banjul, the capital of Ghana, on October 6, 2022.
Milan Berckmans/AFP by way of Getty Images
In December 2022, Nepal blacklisted 16 Indian pharmaceutical companies because authorities claimed that they failed to comply with the WHO quality manufacturing standards. Local brokers have been requested to right away recall the medicine. The Nepalese authorities stated that this transfer got here after they’d despatched a workforce of drug inspectors to India to the manufacturing services of the pharmaceutical corporations that had utilized to export their merchandise to Nepal; these 16 corporations did not make the minimize. The corporations that have been blacklisted haven’t responded to those claims.
On August 11, in Uzbekistan, an Indian nationwide representing the pharma firm Marion Biotech, primarily based in Noida, close to Delhi, was put on trial. The Uzbekistan authorities accused the corporate of distributing contaminated cough syrup that killed 65 children in December 2022 and paying native officers a bribe of $33,000 to skip the nation’s necessary high quality testing. In March, Indian authorities suspended Marion Biotech’s license after a probe revealed that 22 of the corporate’s merchandise have been “not of standard quality” and that the cough syrups they’d exported have been contaminated. The firm has denied the fees.
Such adulteration to chop prices is pretty widespread, the authors of The Truth Pill level out. Indian pharma producers do not take a look at batches as vigilantly as they need to, the authors say.

Wuri Bailo Keita, 33, holds a cell phone exhibiting an image of himself and his late daughter, Fatoumatta, who’s believed to have died of acute kidney failure after ingesting contaminated cough syrup manufactured in India. The household lives in Banjul in The Gambia. The picture is from October 10, 2022.
Milan Berckmans/AFP by way of Getty Images
conceal caption
toggle caption
Milan Berckmans/AFP by way of Getty Images

Wuri Bailo Keita, 33, holds a cell phone exhibiting an image of himself and his late daughter, Fatoumatta, who’s believed to have died of acute kidney failure after ingesting contaminated cough syrup manufactured in India. The household lives in Banjul in The Gambia. The picture is from October 10, 2022.
Milan Berckmans/AFP by way of Getty Images
Propylene glycol, which is an important chemical ingredient in cough syrups, is commonly contaminated with the cheaper ethylene glycol and diethylene glycol utilized in industries as solvents for paints, plastics and antifreeze fluid for brakes. And this may be poisonous.
Then there was the problem of price-fixing.
In the U.S, the Indian pharma big Glenmark admitted guilt and agreed to pay a penalty of $30 million to the U.S. Department of Justice. Glenmark was charged with conspiring with Israeli drug maker Teva to repair the worth of pravastatin, a generic drug for prime ldl cholesterol. Price fixing is unlawful globally as a result of it raises costs of medicine.
But in India itself, prosecution of pharmaceutical corporations is difficult.
Legal loopholes exist in India for these corporations to flee prosecution.
“The Indian parliament passed a bill in the recent monsoon session [on July 27, 2023] that decriminalized two provisions of India’s Drugs & Cosmetics Act. The revised legislation says that those that manufacture medication “that aren’t of normal high quality” could escape jail time by choosing to pay fines —a paltry $200,” says Thakur.
“Reports of contaminated drugs can affect sales, so governments have a financial incentive to step in and protect the companies,” says Reddy.
Even the case of the deaths in Gambia has not introduced a response from India’s authorities. “Gambian officers had done an investigation linking the deaths of the youngsters to the cough drugs manufactured in India. The United States Center for Disease Control’s investigation established a strong link as nicely,” says Dinesh Thakur.
However, India’s Health Minister, Mansukh Mandaviya, categorically denied that the cough syrups have been contaminated and that this was the reason for dying. Speaking with the news agency the ANI, he stated that the reason for the dying was most likely that the youngsters within the Gambia got cough syrup after they’d already been affected by diarrhea.
The Gambia experienced severest flooding in July, and this was linked to a diarrheal outbreak. However, a WHO laboratory analysis of the cough syrups confirmed that they contained “unacceptable amounts of diethylene glycol and ethylene glycol as contaminants,” which when consumed, can show deadly.
A brand new requirement to make use of QR codes on medicine bins to stop distribution of faux medication has been launched since August 1. Critics do not assume it’s going to assist.
In March, the Indian Health Ministry requested India’s Department of Pharmaceuticals to plot an inventory of 300 extensively used drug manufacturers, in order that they might put QR codes (a machine-readable barcode) on the bins. The QR code would guarantee each pharmacists and prospects that the medicine was genuine and that it might be traced again to the corporate that produced it in case of any complaints.
“QR codes on boxes can help identify a drug and prevent counterfeiting,” says Thakur. “But the government’s own data shows that counterfeit (fake) drugs aren’t the problem in India. The bigger problem is the drugs that are not of standard quality.”
In 2016, India’s Central Drugs Standard Control Organization analyzed a complete of 47,012 samples of medicine accessible within the open market. Eight samples have been discovered to be pretend — missing any of the lively chemical elements talked about within the labeled drug. However, 1,011 samples have been deemed “not of standard quality,” which signifies that they’d all of the lively elements however simply not in the best quantities.
The World Health Organization is not prone to step in.
Asked if they’d play an even bigger function in monitoring the standard of medicine from India, the World Health Organization gave this response to NPR from Rutendo Kuwana, workforce lead, Incidents and Substandard/Falsified Medical Products: “WHO is not an agency that prosecutes but we may liaise with law enforcement agencies if it is in the interest of protecting public health. Also, we do support countries to maintain their own high standards. We facilitate collaboration through something called the member state mechanism. This will address the issue of tackling substandard and falsified medical products in a transparent, inclusive way. We also run regional workshops/meetings to train and discuss this work. Our alerts are one way to bring awareness, support action and protect people plus our guidelines provide a framework for countries to build their own systems of cooperation.”
A scarcity of a world regulatory framework for pharmaceutical high quality is sorely felt, the authors say, as a result of these corporations can’t be held to account for the hurt they trigger, both by their very own governments or by a world physique such because the WHO.
“The WHO says it’s voluntary compliance — up to the buyer and the seller. There are no consequences if something goes wrong,” says Thakur. “And that is a problem.”
Kamala Thiagarajan is a contract journalist primarily based in Madurai, Southern India. She experiences on world well being, science, and growth, and her work has been revealed within the New York Times, The British Medical Journal, BBC, The Guardian and different shops. You can discover her on twitter @kamal_t
[adinserter block=”4″]
[ad_2]
Source link
